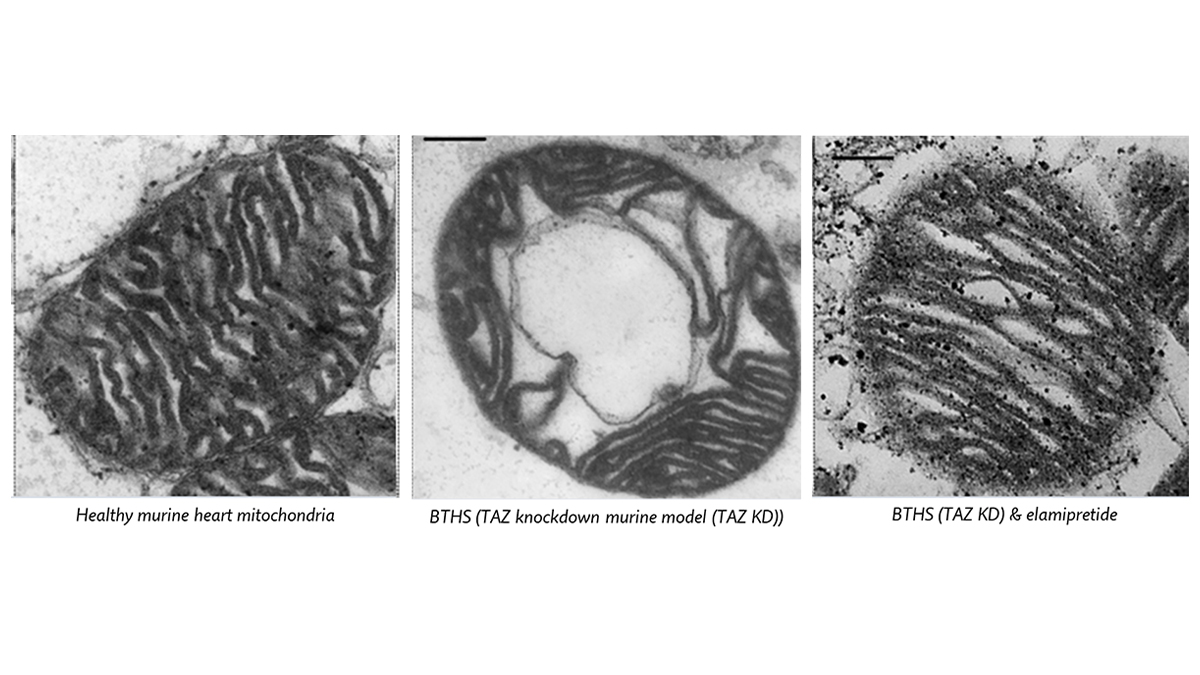
“TEM images of cardiac mitochondria isolated from healthy mice (left panel), Barth syndrome murine model without treatment (centre panel) and with elamipretide treatment (right panel) show how elamipretide mitigates severe alteration of morphology in TAZ-deficient mice.” (These images are modified by Russo et al. 2024.)
First Drug Approved to Directly Treat Mitochondria
Stealth BioTherapeutics, Inc, (Stealth) has received FDA approval for its drug FORZINITY™ (Elamipretide Injection). The drug is used to improve muscle strength in adult and pediatric patients over 30 kg with Barth syndrome, a rare and life-limiting disease. FORZINITY is the very first mitochondrial-targeting therapy approval.
“FDA approval culminates more than a decade of clinical development,” said Reenie McCarthy, Stealth CEO. “We were inspired by patient advocacy to start our development journey and patients remained our north star along the way, strengthening our resolve through every challenge.”
Barth syndrome is a life-limiting pediatric disease that affects only about 150 people in the United States. The disease is caused by a mutation in the TAFAZZIN (TAZ) gene that results in the loss of tetralinoleoyl-cardiolipin, a key lipid for mitochondria. It is an x-linked disease, which means that females can be carriers, but typically only males are affected. Patients suffer from exercise intolerance, muscle weakness, debilitating fatigue, heart failure, recurrent infections, and delayed growth. The greatest risk of death occurs before age 5.
Mitochondria are tiny cell organelles primarily known for producing energy within the cell, but they also have many other important activities. Each cell contains hundreds to thousands of mitochondria. Mitochondria are also unique in that they contain their own DNA. They are increasingly implicated in many diseases, but, to date, there have been no successful drug interventions of the mitochondria themselves.
“We are grateful for and applaud this incredible step that was reached expeditiously by the FDA after Stealth’s most recent new drug application and after years of circuitous challenges,” said Emily Milligan, MPH, executive director of Barth Syndrome Foundation (BSF). “We have worked tirelessly to support this outcome, and today is a day to celebrate, although much work remains.”
Elamipretide is reported to show improvement in mitochondrial structure and function in a cardiolipin-dependent manner, resulting in improved heart and skeletal muscle function 2-5. It readily enters mitochondria and migrates to the mitochondrial inner membrane 2,6. Once there, it specifically binds to cardiolipin to improve membrane stability, enhance ATP synthesis, and reduce the production of reactive oxygen species.
Simona Lobasso, PhD, an expert on lipidomics and cardiolipin role at the University of Bari, Italy, recently reported 4 that Elamipretide treatment improves ultrastructural morphology (i.e., inner membrane and cristae) and function in isolated cardiac mitochondria by restoring their ability to recycle themselves within the heart cells in a mouse model of Barth syndrome.
She added, “In a previous study,4 we found that in vivo treatment of TAZ-deficient mice with Elamipretide promoted respiratory “supercomplex” organization in cardiac mitochondria. We hypothesize that it exerts its beneficial effect by entering mitochondria and influencing the function of the respiratory chain. By improving mitochondrial structure, respiratory capacity and dynamics, Elamipretide can also improve overall heart and skeletal muscle functions in treated mice.”
Michael Murphy, PhD, a noted mitochondrial expert at the University of Cambridge who is not associated with Elamipretide or its approval process, but familiar with clinical trials and FDA drug approval offered, “The approval of Elamipretide for Barth syndrome patients arose from an open label 168-week trial of 10 patients with eight completing the trial with functional improvements being reported.” He added, “This is a potentially good step for this group of patients who have few treatment options. While this outcome cannot be compared with a long-term placebo-controlled double-blind study, the small number of patients and their prognosis make this challenging.”
Such breakthroughs are critical for the support foundations. “BSF will continue its efforts to advocate for label expansion to include individuals under 66 pounds and monitor Stealth’s progress on meeting post-approval requirements and coordinating with our international affiliates to expand access internationally.” said Lindsay Marjoram, PhD, BSF director of research.
“While we are thrilled to have achieved this milestone, we are keenly aware that our work is not done,” said Jim Carr, Stealth chief clinical development officer. “We took the weekend after the approval to celebrate and then leaned right back in to initiate activities for our post-marketing trial, which will enroll 48 subjects, age 5 and older, at sites in Europe and Australia. We also plan to meet with the FDA in the next few months to align on a pathway toward expanding the label to include younger children.”
Stealth plans to continue working on elamipretide for additional indications, including children less than 30 kg with Barth syndrome and dry age-related macular degeneration and primary mitochondrial myopathy. In addition, they are developing bevemipretide for ophthalmic and neurological disease indications.
Questions for about the approval process and the prospects for the disease:
MitoWorld: FDA approval of elamipretide is a landmark achievement. Do you think this will encourage other pharm and biotech companies to pursue therapies for these rare diseases?
Lindsay T. Marjoram (LTM): It is our hope that this approval will help kickstart future investments into both mitochondrial and ultra-rare diseases. I think this approval helps to demonstrate that the FDA is more committed to using the accelerated approval pathway in ultra-rare drug submissions. To spur future investment, though, it will be critical for Congress to reauthorize the Pediatric Priority Review Voucher Program, which gives companies, such as Stealth, the financial incentive to invest in diseases that may not be profitable.
MitoWorld: Can you elaborate on the molecular mechanism of elamipretide?
LTM: Elamipretide is a mitochondrial cardiolipin binder that localizes to the inner mitochondrial membrane and improves mitochondrial morphology and function. In Barth syndrome, lack of TAFAZZIN activity leads to a build-up of immature (monolyso-) cardiolipin and alters the structure of the inner membrane. The impaired structure leads to decreased energy production.
MitoWorld: Barth syndrome is a rare disease. How hard was it to identify study a sufficient number of subjects to obtain significant results?
LTM: BSF was a partner in ensuring that the trial was enrolled. I believe it took less than a year to fully enroll. There were a lot of learnings from this process since it was the very first trial to be run in our community. One thing we learned from this trial was that it needed to run longer than anticipated.
MitoWorld: Are you looking at other drug candidates for mitochondrial diseases?
David Brown, PhD, Stealth senior vice president of discovery: Our next generation clinical stage compound, bevemipretide, is in early clinical trials, and we have a deep pipeline of mitochondrial targeted therapeutics in early stage development.
LTM: BSF has funded >$7M in research, which has resulted in >$41M in follow-on funding from agencies, such as NIH. The Foundation continues to support research into the basic mechanistic underpinnings of Barth syndrome and development of potential therapeutics, including enzyme replacement therapy, AAV-mediated gene therapy, anti-sense oligonucleotide therapy, and small-molecule development.
References
1 U.S. Prescribing Information:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/215244s000lbl.pdf
2 Szeto HH (2014) First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 171: 2029–2050.
3 Chatfield KC, et al. (2019) Elamipretide improves mitochondrial function in the failing human heart. JACC: Basic Transl. Sci. 4: 147–157.
4 Russo S, De Rasmo D, Rossi R, Signorile A, Lobasso S (2024) SS-31 treatment ameliorates cardiac mitochondrial morphology and defective mitophagy in a murine model of Barth syndrome. Sci. Rep. 14: 13655.
5 Russo S, De Rasmo D, Signorile A, Corcelli A, Lobasso S (2022) Beneficial effects of SS-31 peptide on cardiac mitochondrial dysfunction in Tafazzin knockdown mice. Sci Rep. 12: 19847.
6 Sabbah HN, Alder NN, Sparagna GC, Bruce JE, Stauffer BL, Chao LH, Pitceathly RDS, Maack C, Marcinek DJ (2025) Contemporary insights into elamipretide’s mitochondrial mechanism of action and therapeutic effects. Biomedicine & Pharmacotherapy 187: 118056.






