Mitochondria Transfer from Cancer Cells to Fibroblasts
A paper in Nature Cancer by a multi-institute research team, led by Michael Cangkrama and Sabine Werner at the ETH Zurich, describes how cancer cells use the transfer of mitochondria to highjack fibroblasts to support the cancer. The study also implicates a protein as critical for the transfer that may be useful in novel therapeutic strategies.
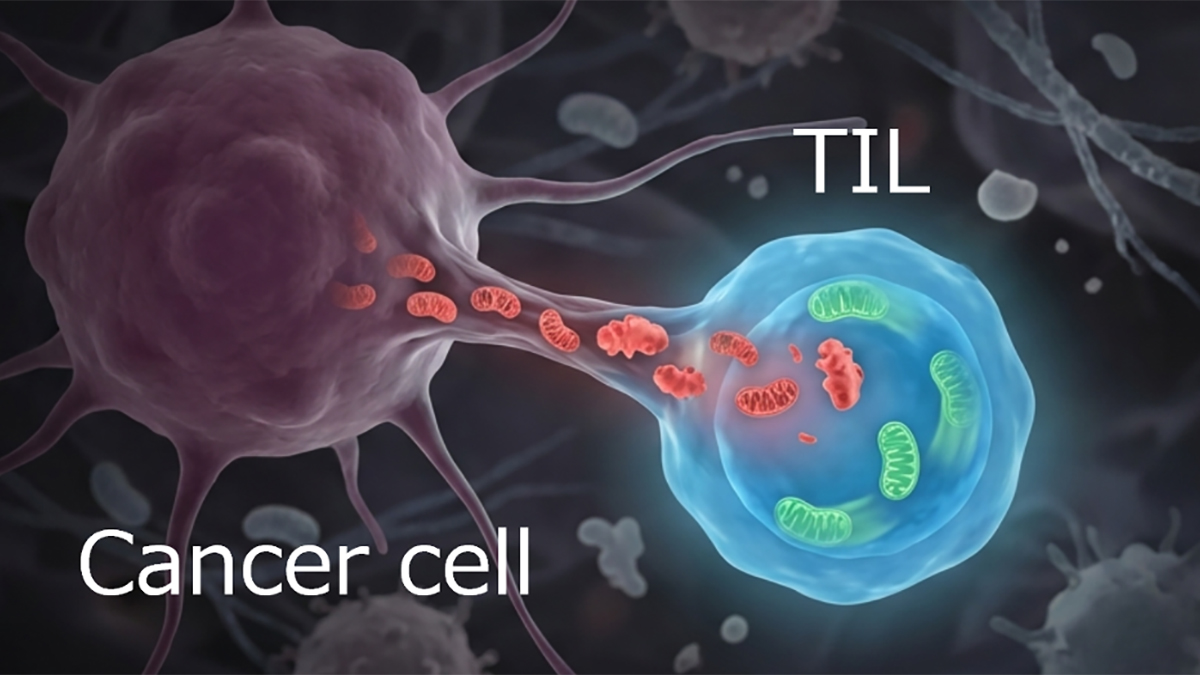
The transfer of mitochondria from various cell types into cancer cells has been reported to enhance cancer cell proliferation, motility and more. Fibroblasts associated with tumors are known to adopt certain characteristics, and they frequently support cancer development. The Cangkrama-Werner team sought to determine if the transfer of mitochondria might work in the opposite direction. In other words, do cancer cells transfer mitochondria to the fibroblasts?
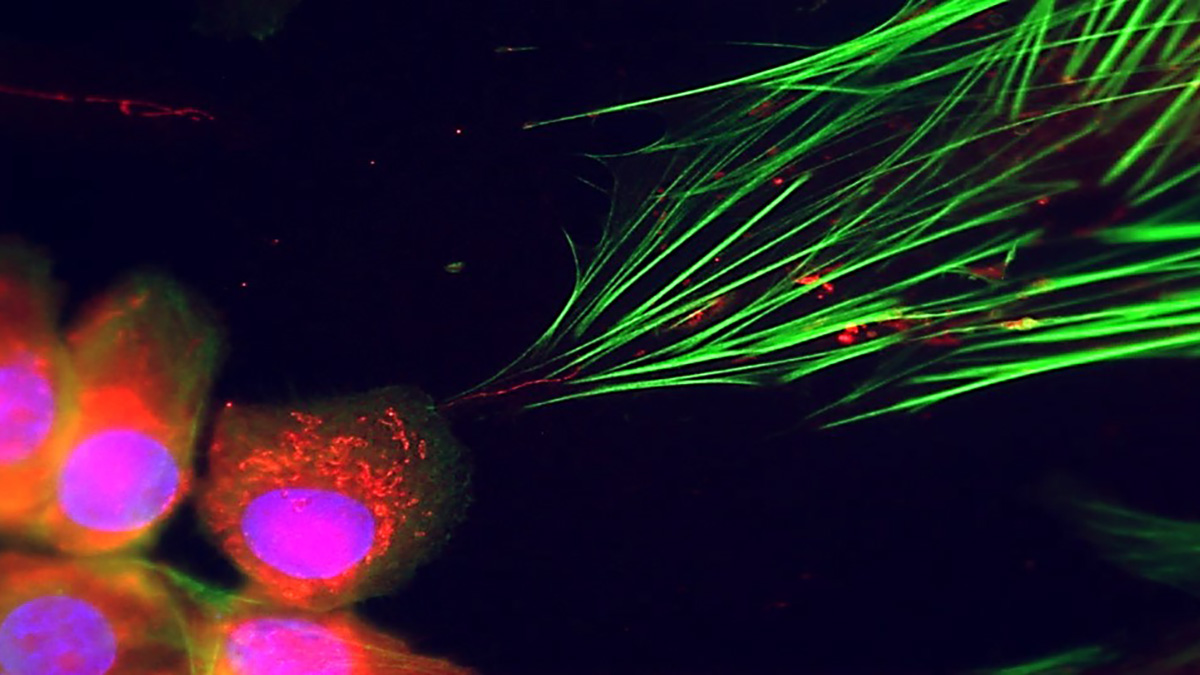
To answer this question, the team examined cancer cells and fibroblasts in co-cultures and xenograft tumors. Intriguingly, mitochondria were transferred to the fibroblasts where they caused changes in the metabolism of the recipient cells. The fibroblasts began to express markers associated with cancer-associated fibroblasts and to release secreted soluble and extracellular matrix proteins that support tumor growth. The team also found that the transfer of mitochondria depends on the mitochondrial trafficking protein MIRO2. Without MIRO2, the transfer and the other changes failed. These findings are consistent with the overexpression of MIRO2 at the leading edge of epithelial skin cancers and other malignancies. Finally, they found that the mitochondria are transferred through thin membranous structures called tunneling nanotubes.
This study demonstrates that mitochondrial transfer from cancer cells to fibroblasts is a key regulator of CAF differentiation. The findings also implicate MIRO2 and mitochondrial transfer as potential targets for strategies to treat cancers.
A conversation with Drs. Cangkrama and Werner
MitoWorld: Can you give us an idea of what studies you might do to advance the findings presented here?
Werner: There are several open issues, including the signals that induce the transfer, the genes and proteins that promote the transfer and the targets of the transferred mitochondria, which induce the pro-tumorigenic phenotype in fibroblasts.
MitoWorld: You showed that oxidative phosphorylation is the main activity that is influenced by the mitochondrial transfers. Can you speculate on how that process translates into the changes in gene expression in the fibroblasts? Might there be other mitochondrial activities involved in activating the interferon-response genes?
Werner: Oxidative phosphorylation is highly relevant in this context, but it is probably not the only relevant factor. Oxidative phosphorylation results in the production of various metabolites, which are likely to regulate transcription factors that control gene expression in fibroblasts. We found that the interferon-response genes are not regulated by transferred mitochondria, but likely by transfer of other molecules from cancer cells to fibroblasts in co-cultures, which remain to be identified.
MitoWorld: The transfer of mitochondria from cell to cell has been somewhat controversial. Can you comment on the seeming reluctance of some researchers to embrace this concept?
Werner: Many results were obtained with MitoTracker, which is known to be leaky. Several studies did not include appropriate controls to address this problem, which might have contributed to skepticism about mitochondrial transfer. The concept was also unexpected when first proposed. Even when transfer occurs, it is difficult to predict and appears to depend on specific conditions, such as cellular stress or high metabolic demand, further fueling doubts about its physiological relevance. However, many exciting and well-controlled studies have recently been published, which show the relevance of mitochondrial transfer under various conditions. This makes mitochondrial transfer a promising area of research, with the potential to uncover new mechanisms of intercellular communication and to develop innovative therapeutic strategies.
MitoWorld: Can you speculate how your findings might translate to therapies for cancer?
Werner: There is a long way from this discovery to a cancer drug. Nevertheless, our work suggests that inhibition of mitochondrial transfer (e.g., by blocking MIRO2 or other proteins important for the transfer) could be a novel approach for cancer treatment. In addition, identification of the relevant targets of the mitochondrial transfer in recipient fibroblasts could provide new therapeutic opportunities.
MitoWorld: How did you first become interested in mitochondria?
Werner: Several previous studies of our laboratory revealed the important role of mitochondria in the pro-tumorigenic cancer-associated fibroblast phenotype. This raised our interest in these exciting organelles.
Reference
Cangkrama M, Liu H, Wu X, Yates J, Whipman J, Gäbelein CG, Matsushita M, Ferrarese L, Sander S, Castro-Giner F, Asawa S, Sznurkowska MK, Kopf M, Dengjel J, Boeva V, Aceto N, Vorholt JA, Werner S (2025) MIRO2-mediated mitochondrial transfer from cancer cells induces cancer-associated fibroblast differentiation. Nature Cancer 6: 1714–1733.