The Nerve of Some Cancers
Do neurons help cancer spread? In a paper published in Nature, a multi-institution research team, led by Simon Grelet at the University of South Alabama, provides strong evidence for a key relationship between cancer cells and neurons. They showed that mitochondria migrate from neurons to cancer cells to increase the metabolism of the cancer cells.
Dr. Grelet’s team focused on the mitochondria in cancer cells. The spread of tumors requires a significant amount of energy, and mitochondria produce energy for the cells. Cancer cells are well-known for adapting their metabolism to fit changing conditions. However, this metabolic plasticity was thought to be due to changes within the tumor cells themselves. For example, they could obtain energy by glycolysis (a less efficient process for producing ATP in the cytoplasm) or by oxidative phosphorylation (a more efficient process in the mitochondria). However, cancer cells also receive outside help in the form of metabolites, growth factors and cytokines from other cells.
Previous experiments had shown that somehow the neurons aid tumor progress, but what really interested the Grelet team was a relatively new concept that mitochondria can move from cell to cell. When neurons were co-cultured with tumor cells, the neurons experience changes to their metabolism. The number of their mitochondria increases, and some are transferred to the tumor cells. How could this work?
The team first looked at a co-culture of an aggressive breast cancer cells and neurons. The neurons underwent a morphological change from globular to tubular structures, which indicated the cancer-induced differentiation of the neurons. Neuronal differentiation is associated with changes of metabolic programming from glycolysis to oxidative phosphorylation. These changes were also associated with the establishment of long neuron protrusions, forming a neural network in the culture in vitro, which reflects the establishment of a nerve-cancer crosstalk and suggests the communication or sharing of biological materials.
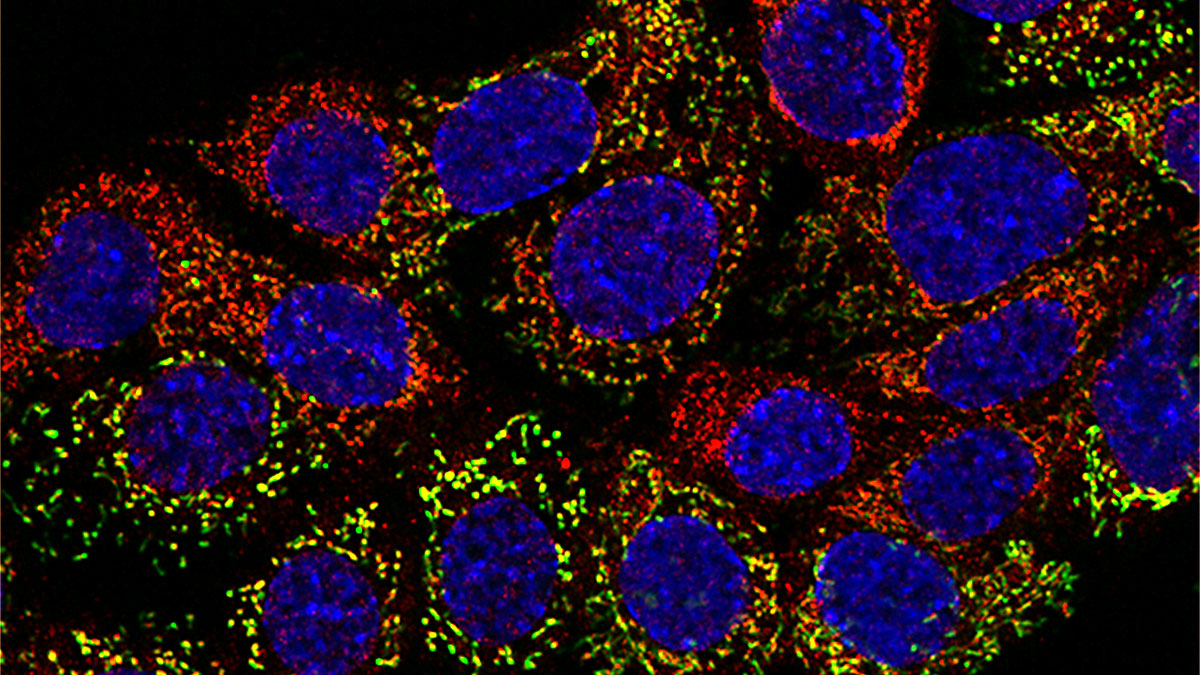
Next, the team used fluorescent-labeled mitochondria to show that the mitochondria migrated from neurons to cancer cells. These methods revealed additional insights into the transfers, but they had significant limitations. To overcome those limitations, the team developed MitoTRACER. This system used genetic engineering where the donor cell mitochondria carry a tag protein that, once transferred to the recipient cells, triggers an enzymatic reaction activating the permanent expression of a fluorescent protein. The trick is that, when mitochondria move from the neurons to the cancer cells, the red fluorescence is lost, and a green signal is permanently activated. Thus, it is easy to monitor cells with mitochondria that have migrated into a new cell.
Using this unique approach, the researchers have been able to investigate the fate of the recipient cells during the cancer progression cascade, and by fate mapping of the recipient cells from the primary tumor during metastasis progression in vivo. The researchers noted enrichment of acquired mitochondria in brain metastases that might indicate enhanced metabolic plasticity from those extra mitochondria. Neuronal mitochondria are more metabolically active and might better enable tumor cells to thrive in the brain environment. This approach allowed us to define the role of mitochondrial transfer in the primary tumor environment. This process generates a subset of highly efficient metastasis cells that succeed through the complex stops and that are resilient to the metastatic barriers to ultimately form distant metastases, which is the main driver of cancer-associated mortality.
In summary, this fascinating paper might have significant implications for future cancer therapies, and beyond the cancer context, the mitoTRACER approach could have broader applications in studying cell-cell transfer of mitochondria in health and disease to understand the physiopathological implications of these transfers better.
Discussion with Dr. Grelet
MitoWorld: Your paper brings to mind the work of Judah Folkman who showed that tumors need and encourage the formation of a blood supply to progress. What do you believe the tumor may be getting from the nerve cells?
Interesting comparison. Yes, there are definitely commonalities between cancer angiogenesis and cancer innervation. In fact, cancer can actively “call” neurons to innervate them. In the context of cancer, we recently showed that aggressive subtypes of breast cancer cells can secrete axon guidance molecules, including Semaphorin-4F, to promote cancer innervation. We also found that this increased nerve density is associated with enhanced metastasis (PMID: 34810279).
The role of axon guidance molecules in promoting cancer innervation was demonstrated years ago by Dr. Gustavo Ayala (PMID: 11746267; PMID: 24097862), who pioneered the field of cancer innervation and collaborated with us on this study. Since then, many studies, including our own, have confirmed the contribution of these molecules to cancer innervation and cancer aggressiveness. While increased nerve density is often associated with poor clinical outcomes, the underlying mechanisms have remained poorly understood. This gap in understanding is precisely what motivated us to conduct this study.
MitoWorld: The movement of mitochondria from cell to cell is still controversial. What do you think it will take to solidify the concept?
The idea of intercellular mitochondrial transfer remains controversial, largely due to the lack of robust tools and clear in vivo evidence. To solidify the concept, new rigorous and physiologically relevant approaches are needed. For example, genetic systems, such as our MitoTRACER approach, which allow for precise and conditional signaling of transfer events, open the avenue for lineage-based tracking of transferred cells in vivo. Such models are critical to move the field forward and to convincingly demonstrate that mitochondrial transfer is not merely an artifact, but a biologically meaningful process.
MitoWorld: How do you think the cells signal to each other to initiate the transfer of mitochondria?
A variety of signals could potentially be involved in initiating mitochondrial transfer, but these remain to be better defined. In the context of cancer innervation, axon guidance molecules may contribute; more generally, transfer events may occur as part of a stress or help response, or by hijacking existing physiological communication routes. These signals could include reactive oxygen species, cytokines, or changes in membrane potential or lipid composition. Cancer cells, for instance, can upregulate molecules involved in cell-cell contact to facilitate mitochondrial transfer, as elegantly demonstrated by Watson et al. (PMID: 37169842). Deciphering the molecular language of this intercellular request remains a critical next step for the field.
MitoWorld: These experiments seem to have ramifications for how the nuclear and mitochondrial genomes coordinate their activities. Do you think the communication between the donated mitochondria and the recipient cell also involves the donor genes?
Absolutely, and this is a topic that warrants further investigation. Mitochondrial transfer introduces not only a new metabolic organelle with all its associated machinery, but also foreign mitochondrial DNA into the recipient cell, raising important questions about nuclear–mitochondrial coordination. While the nuclear genome of the recipient cell continues to govern most mitochondrial functions, the donor mitochondria carry their own genome and organelle machinery, which in our case is fine-tuned for neuronal metabolism and may not be fully compatible. This mismatch could influence mitochondrial gene expression, protein stoichiometry, and ultimately, the bioenergetic output. Whether donor mitochondrial DNA or its transcripts actively modulate recipient cell behavior remains an open and fascinating question. Clarifying the extent and mechanisms of this intergenomic crosstalk is essential to fully understand the consequences of mitochondrial transfer.
MitoWorld: You note that more work is needed to determine if the effects of the donated mitochondria are related to metabolic efficiency or simply that there are more of them. Do you have any sense of which it might be?
In the context of neurons, it is likely a combination of both abundance and quality. On one hand, the increase in mitochondrial mass may help support basic energy demands, particularly under stress. On the other hand, the quality and origin of the donated mitochondria, such as their neuronal bioenergetic efficiency, may actively contribute to metabolic reprogramming in the recipient cell. In our system, the integration of neuronal mitochondria appears to enhance not only energy production but also metabolic plasticity, enabling cancer cells to better adapt to new microenvironments. Dissecting the relative roles of mitochondrial abundance versus functional impact will be key to understanding how mitochondrial transfer influences cell fate and behavior.
MitoWorld: Folkman used his findings to suggest that cancers could be treated by preventing angiogenesis, and that is now widely accepted. Do you have any thoughts on how your findings with neurons might be used to develop therapeutic strategies for cancer?
Yes, absolutely, that is the ultimate goal. There are several possible strategies. First, we can target tumor innervation itself, which supports cancer progression. Second, we can aim to block the intercellular transfer of mitochondria, which fuels cancer cell adaptation. Third, and perhaps most compelling, we can selectively target recipient cancer cells that have acquired unique metabolic or phenotypic traits as a result of mitochondrial uptake. These cells may represent a vulnerable subpopulation that could be eliminated through precision therapies. Together, these approaches offer a new framework for tackling cancer’s adaptability.
MitoWorld: You looked at breast cancer and melanoma cells, both highly metastatic cancer types. Is the neuronal connection equally valid in less metastatic cancers?
We also previously reported a link between cancer plasticity and cancer innervation (PMID:37046688). Our findings suggest that more aggressive cancer cells are more likely to establish neuronal connections and engage in mitochondrial transfers. This, in turn, may further amplify their aggressive behavior, creating a vicious cycle. The ability of these cells to co-opt neural signaling and reshape their metabolic and phenotypic programs reflects a high degree of plasticity that likely contributes to disease progression.
MitoWorld: Also your work focused on solid tumors. Is there anything to be learned about blood tumors?
Interesting point. Blood cancers reside in richly innervated niches, such as the bone marrow, where neurons regulate hematopoiesis and immune responses. So, similar mechanisms might shape the behavior of leukemia or lymphoma cells. However, this remains largely unexplored, and adapting lineage-tracing tools, such as MitoTRACER, to hematological models could open exciting new directions in the field.
Reference
Hoover G, Gilbert S, Curley O, Obellianne C, Lin MT, Hixson W, Pierce TW, Andrews JF, Alexeyev MF. Ding Y, Bu P, Behbod F, Medina D, Chang JT, Ayala G, Grelet S (2025) Nerve-to-cancer transfer of mitochondria during cancer metastasis. Nature