Mitochondrial Microscopist Dane Wolf Launches MitoGraphica
We are happy to announce that Dr. Dane M. Wolf, recently an EMBO Long-Term Fellow at the University of Cambridge, is starting his own mitochondrial microscopy consultancy and will serve as MitoWorld’s Director of Microscopy.
Wolf is a mitochondrial biologist specializing in advanced imaging technologies. He completed his PhD at Boston University and University of California, Los Angeles, where he used live-cell super-resolution microscopy to study the structure-function relationship of mitochondrial cristae. As an EMBO Long-Term Fellow at the University of Cambridge, he investigated mitochondrial (mt)DNA dynamics using cutting-edge imaging approaches.
Wolf founded MitoGraphica, a scientific consultancy focused on mitochondrial image analysis and research, to support both academia and industry. He is deeply passionate about mitochondria and is committed to advancing and communicating our understanding of their vital roles in human health and longevity.
“In my lab, Dane became an imaging virtuoso,” said Dr. Orian Shirihai, Wolf’s PhD mentor, “not just because of his technical skill, but because of his creativity and attention to detail. What he’s built with MitoGraphica is a natural extension of his scientific work: a consultancy grounded in rigor, reliability, and precision. He brings not only innovation and vision, but also the discipline to deliver timely, well-documented, statistically sound solutions to complex image analysis challenges—something the field truly needs.”
MitoWorld: You assembled a nice slide deck (download PPTX) tour of some of the imagery you produced while at UCLA. Can you say a little bit about what we see in that deck?
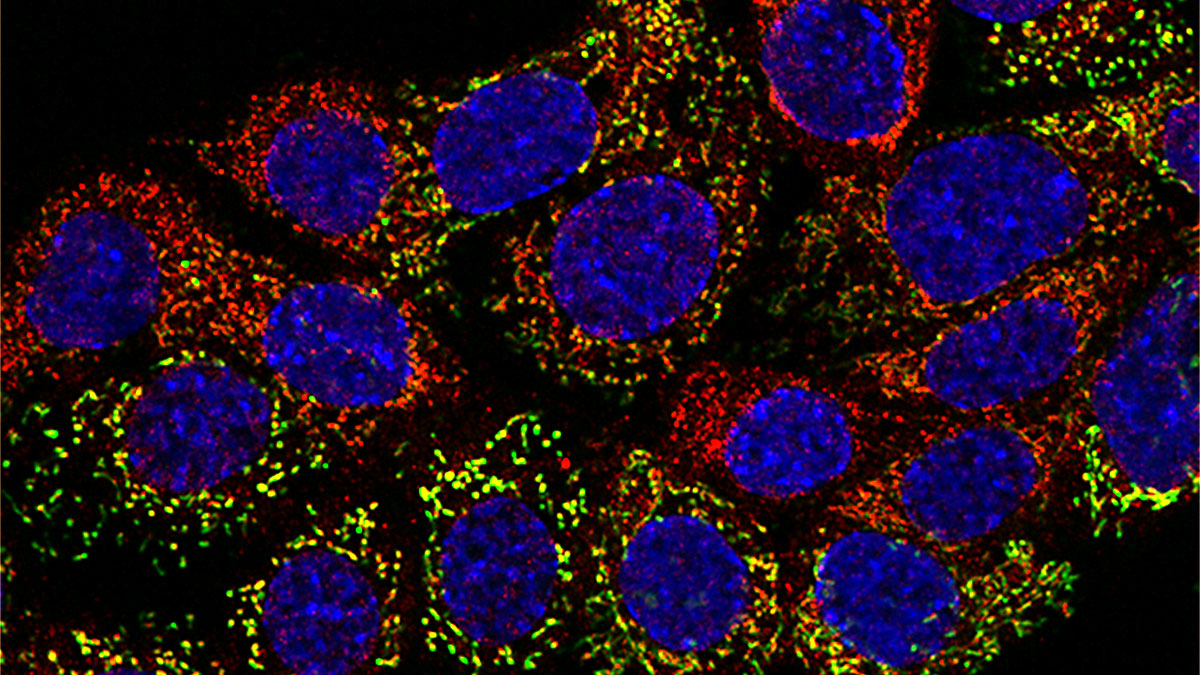
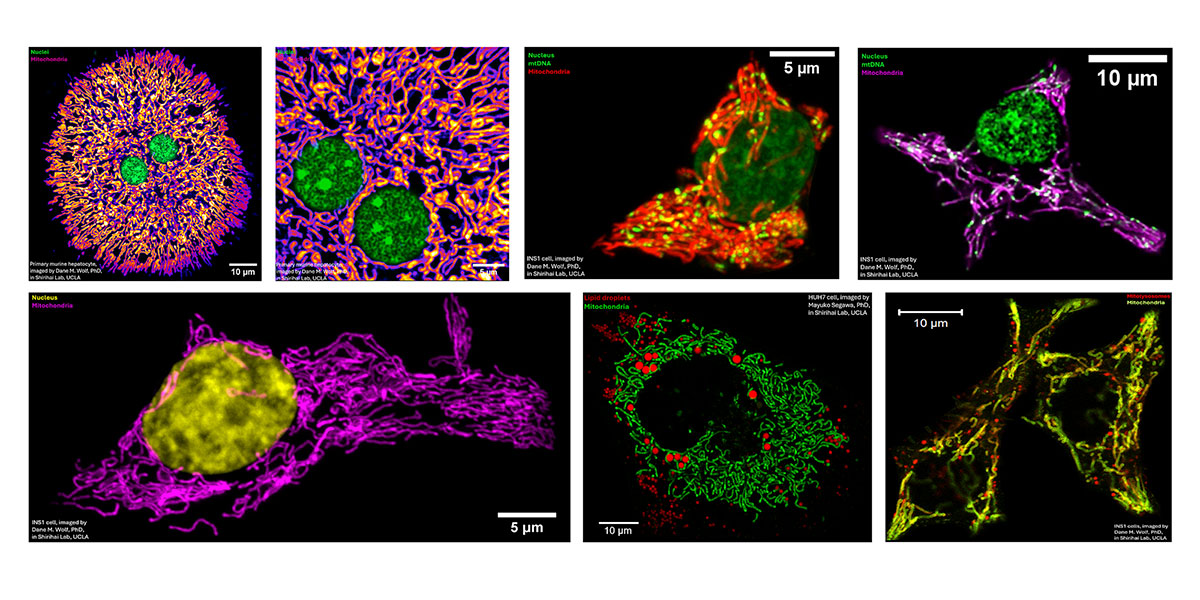
Wolf: It’s an overview of the imaging that I performed at UCLA. The lab focused not only on the role of mitochondria in single cells, but also on how they regulate metabolism across the body. I spent a lot of time imaging a pancreatic β cell line (INS-1) and primary hepatocytes—which carry out the bulk of the liver’s metabolic activity. The deck also includes images of antibody-producing B cells undergoing mitosis in response to a stimulatory signal, along with super-resolution imaging of cristae, lipid droplets, lysosomes, and mtDNA in a variety of cell types.
The main takeaway from this set of images is that virtually all eukaryotic cells are densely populated with mitochondria. They inhabit the cell almost like individuals in a village—exhibiting complex behaviors, being born and dying, in a manner of speaking—and recent work suggests they take on specialized roles within the broader cellular environment. Remarkably, mitochondria can even travel between cells via membranous bridges or vesicles, coming to the aid of stressed neighbors.
MitoWorld: Which came first, your interest in mitochondria or imaging? How did the two evolve together for your work at UCLA and then Cambridge?
Wolf: I’ve been interested in the biology of aging for as long as I can remember. Since mitochondria are central to cellular homeostasis—and their dysfunction is closely linked to the systemic decline associated with aging—my interest in mitochondria came first. That said, it wasn’t until I began my PhD and saw live mitochondria under a confocal microscope that this interest grew from an abstract, academic curiosity into something visceral and compelling. There’s something truly captivating about looking through a microscope and watching thousands of mitochondria wiggle and dart around a field of cells, sometimes undergoing fusion and fission. In those moments, mitochondria seem to exhibit their own kind of behavior. That sense of wonder made me want to follow them more closely, and I became increasingly focused on developing approaches that would allow me to visualize them in greater spatiotemporal detail. The more clearly you can see them— really scrutinize their movements—the more their inner life begins to emerge, in surprising and often beautiful ways.
MitoWorld: During your PhD at UCLA in the Shirihai Lab, what were you trying to accomplish with imaging that you or the lab thought was beneficial, and how did this work add to the field of mitochondrial microscopy?
Wolf: In Orian’s lab, there has always been a strong emphasis on mitochondrial dynamics—how mitochondria move, divide, and fuse. As a PhD student, I was fortunate to have a great deal of freedom to explore, and at UCLA, we had access to cutting-edge super-resolution imaging technologies. One of these systems, Airyscan, allowed us to visualize mitochondria beyond the optical diffraction limit, opening up new possibilities for studying their complex internal structures in live cells.
By optimizing this technology, we developed methods to resolve the inner mitochondrial membrane in living cells. Using membrane-potential-sensitive dyes, we discovered that cristae—the characteristic folds of the inner membrane—were more polarized than other regions, and that individual cristae within the same mitochondrion could display distinct membrane-potential signatures. This supported a new model of the proton motive force, in which the electrical potential that drives ATP synthesis is compartmentalized at individual cristae. In this model, each crista functions like a miniature battery within the larger mitochondrion—much like how a Tesla battery pack is made up of many smaller battery cells (see Wolf et al., 2019).
This level of compartmentalization is thought to enhance energy efficiency. Just as importantly, it provides a form of bioenergetic buffering—if one crista becomes damaged or dysfunctional, it doesn’t compromise the entire organelle. Crista-level autonomy hadn’t been fully appreciated before, and this work added a new layer of understanding to mitochondrial resilience and function.
MitoWorld: Can you briefly describe the range of imaging techniques that you used to investigate mitochondria and how they aid in fundamental research?
Wolf: The imaging techniques that we used are primarily Airyscan and structured illumination microscopy (SIM), two different types of extended or super-resolution microscope. Airyscan employs a 32-channel GaAsP detector array in place of a traditional single-point detector, allowing it to capture more spatial information from the Airy disk of emitted light. Each detector element collects light from a slightly different position, and the combined data are computationally reconstructed to enhance both resolution and signal-to-noise ratio. This approach enables imaging beyond the diffraction limit, down to approximately 120 nm in the x-y plane. Airyscan is also highly sensitive, allowing us to image mitochondria using relatively low laser power. This reduced phototoxicity was critical for measuring membrane potential, as mitochondria are prone to depolarization when exposed to laser-induced stress.
SIM, by contrast, works by projecting striped patterns of light onto a sample from multiple angles and phases. These patterns create moiré interference with fine cellular structures, effectively shifting high-resolution information into a detectable range. By capturing a series of these patterned images and applying computational reconstruction, SIM achieves resolution beyond the optical diffraction limit—down to approximately 120 nm or roughly twice the resolution of conventional confocal microscopy.
Without super-resolution techniques, we wouldn’t be able to resolve the fine details of mitochondrial architecture without resorting to electron microscopy, which requires fixation or freezing, ruling out real-time measurements of structure and function. To truly understand something, you have to see how it moves. Super-resolution microscopy allows us to visualize the dynamics of mitochondria’s innermost structures, shedding light on core aspects of their function.
MitoWorld: What other works of yours might be forthcoming?
Wolf: In many ways, my work in Cambridge built upon the skill set I developed at UCLA. I applied a combination of super-resolution and 4D imaging to investigate the dynamics of the mitochondrial genome. This work is still unpublished, so I can’t go into detail just yet, but I’m looking forward to sharing it with the community, as I believe it offers new insights into fundamental aspects of mitochondrial function.
MitoWorld: Where do you think the field will go over the next 5 years? What do you hope to perfect and investigate over this time?
Wolf: Naturally, it is difficult to predict where the field will go. But if I had to guess, I’d say mitochondrial biology will become increasingly integrated with other disciplines. There’s a growing recognition that mitochondria are fundamental to a wide range of cellular functions, and mitochondrial dysfunction has emerged as a hallmark of an expanding list of diseases.
We’ve known since the mid-20th century that mitochondria are the cell’s powerhouses. A conceptual leap at the end of that century was the realization that they also play a central role in determining whether a cell lives or dies. The 21st century has brought about a renaissance in mitochondrial biology, with new discoveries revealing their involvement in diverse physiological contexts—from maintaining stem cell identity to driving specific cancer behaviors through shifts in metabolic activity.
I’m particularly interested in the role of mitochondria in the aging process. Finding new ways to preserve or rescue mitochondrial function, especially under stress, will likely be essential in efforts to combat age-related diseases such as neurodegeneration. And of course, I am always working to stay at the forefront of imaging technology, as visualizing mitochondrial behavior in ever-increasing detail continues to unlock new insights.
MitoWorld: How do you hope to shape your business, and what services can you bring to labs to improve or design their microscopy for cutting-edge research?
Wolf: After studying mitochondria for over a decade, I’ve come to appreciate that mitochondrial shape is a powerful indicator of function. This isn’t surprising—structure and function are fundamentally intertwined. Understanding how and why mitochondria change shape can reveal important clues about their physiological state, which in turn affects cellular and even organismal homeostasis.
That said, accurately measuring mitochondrial morphology is far from trivial. It requires not only high-quality imaging, but also the expertise to use specialized analysis platforms capable of segmenting and quantifying complex mitochondrial networks. Interpreting these changes—and uncovering the mechanisms behind them—can be especially challenging for researchers who aren’t specialists in mitochondrial dynamics or function.
Given the growing interest in mitochondria across both industry and academia, I founded www.MitoGraphica.com to make this expertise more accessible. The goal is to support researchers who want to image mitochondria but need guidance, as well as those who already have images but require expert help with analysis and interpretation. Ultimately, progress in both basic biology and therapeutic development depends on rigorous experimental design and accurate data. I want MitoGraphica to be a reliable resource—ensuring that anyone studying mitochondrial function has access to the tools, insights, and support needed to do it well.